Multiple-Choice Items:
1. Which statement best describes a supersaturated solution?
|
A
|
A solution that is able to dissolve more solute at a given temperature
|
|
B
|
A solution with more solute dissolved in the solution than its saturation point allows
|
|
C
|
A solution that has the maximum amount of dissolved solute at a given temperature
|
|
D
|
A solution that has an equal amount of solute and solvent dissolved at a given temperature
|
Use the graph below to answer questions 2–4.
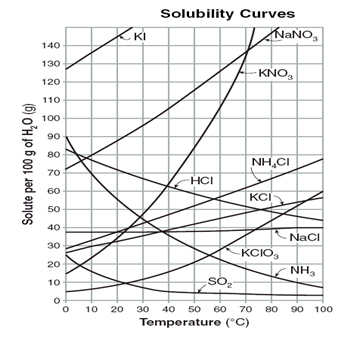
2. How many grams of KNO3 can dissolve into 100 g H2O at 50°C?
|
A
|
75 g
|
|
B
|
80 g
|
|
C
|
85 g
|
|
D
|
90 g
|
3. Which salt is supersaturated when 10 g are dissolved at 10°C?
|
A
|
KlCO3
|
|
B
|
NaCl
|
|
C
|
NaNO3
|
|
D
|
NH3
|
4. Which salt has a saturation point of 55 g at 20°C in 100 g H2O?
|
A
|
KlCO3
|
|
B
|
NaCl
|
|
C
|
NaNO3
|
|
D
|
NH3
|
5. Which statement best describes the relationship between the solubility of solids in a liquid solution and temperature?
|
A
|
As temperature increases, solubility increases.
|
|
B
|
As temperature increases, solubility decreases.
|
|
C
|
As temperature increases, solubility is unaffected.
|
|
D
|
As temperature increases, solubility increases by a factor of 3.
|
6. What two factors can increase the solubility of a gas in a liquid solution?
|
A
|
An increase in temperature and pressure
|
|
B
|
A decrease in temperature and pressure
|
|
C
|
An increase in temperature and a decrease in pressure
|
|
D
|
A decrease in temperature and an increase in pressure
|
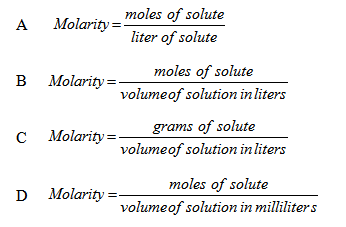
7. What is the formula for the molarity of a solution?
8. A scientist dissolves a type of salt into 100 g of distilled water until no more will dissolve. The scientist looked up the solubility curve for this particular salt and found out 10 g more of the salt was dissolved into the water than the solubility curve suggested was possible. What explanation best explains why the solubility of this solution was greater?
|
A
|
The amount of H2O was less than 100 g.
|
|
B
|
The scientist used a different salt by mistake.
|
|
C
|
The water was a higher temperature than the solubility curve suggested.
|
|
D
|
The scientist stirred the mixture, which caused the intermolecular bonds to strengthen between the salt crystals.
|
9. Which statement best describes the difference between a solution’s molarity and its molality?
|
A
|
Molarity is equal to the moles of solute per liter of solvent; molality is equal to the moles of solute per kilogram of solvent.
|
|
B
|
Molarity is equal to the moles of solute per liter of solvent; molality is equal to the moles of solute per kilogram of solution.
|
|
C
|
Molarity is equal to the moles of solute per liter of solution; molality is equal to the moles of solute per kilogram of solvent.
|
|
D
|
Molarity is equal to the moles of solute per liter of solution; molality is equal to the moles of solute per kilogram of solution.
|
Multiple-Choice Answer Key:
|
1. B
|
2. C
|
3. A
|
4. D
|
5. A
|
|
6. D
|
7. B
|
8. C
|
9. C
|
|
Short-Answer Items:
10. Create a chemical equation representing 8 g of NaCl being dissolved into 100 g of distilled water to create a salt water solution. Label the following on the chemical equation: solute, solvent, solution.
11. What is the prominent gas in soda and what are two ways to increase the solubility of that gas in the soda?
Short-Answer Key and Scoring Rubrics:
10. Create a chemical equation representing 8 g of NaCl being dissolved into 100 g of distilled water to create a salt water solution. Label the following on the chemical equation: solute, solvent, solution.
|
Points
|
Description
|
|
3
|
Student completes all three of the objectives for full credit:
- Chemical equation is accurately written as:
NaCl + H2O = salt water
- Units are labeled accurately and the sum of the solution is accurately written as:
8g NaCl + 100 g H2O = 108g of salt water
- Formula is labeled correctly with the provided terms:
8g NaCl (solute) + 100 g H2O (solvent) = 108g of salt water (solution)
|
|
2
|
Student completes two of the objectives.
|
|
1
|
Student completes one of the objectives.
|
|
0
|
Student completes none of the objectives or fails to attempt the task.
|
11. What is the prominent gas in soda and what are two ways to increase the solubility of that gas in the soda?
|
Points
|
Description
|
|
2
|
Student completes both of the objectives for full credit:
- Student identifies carbon dioxide (CO2) as the gas in soda.
- Student identifies a decrease in temperature and an increase in pressure as ways to increase the solubility of the gas in soda.
|
|
1
|
Student either correctly identifies the gas in soda or identifies both ways to increase solubility of gas in soda, but not both.
|
|
0
|
Student completes none of the objectives or fails to attempt the task.
|
Performance Assessment:
Use the Performance Assessment Lab handout (S-C-9_Performance Assessment Lab and KEY.doc). Prepare the lab ahead of time and hand out copies of the lab sheets to each student.
Safety Note: Goggles, gloves, and aprons must be worn throughout the lab. Please follow your school safety guidelines for handling chemicals.
- Eye contact: Flush the eye with plenty of water. If irritation persists, call for medical help.
- Skin contact: Wash off with water.
- If swallowed: Call for medical help.
Disposal: Small amounts of dilute sodium thiosulfate solution can be flushed down a sink with a large quantity of water, unless local rules prohibit this. Larger amounts of solution or solid should be stored for later disposal. Check local rules before disposing of this chemical.
Source: http://cartwright.chem.ox.ac.uk/hsci/chemicals/sodium_thiosulphate.html
Performance Assessment Scoring Rubric:
See Answer Key in the Performance Assessment Lab (S-C-9_Performance Assessment Lab and KEY.doc).
